Trainspotting within the cell’s antenna
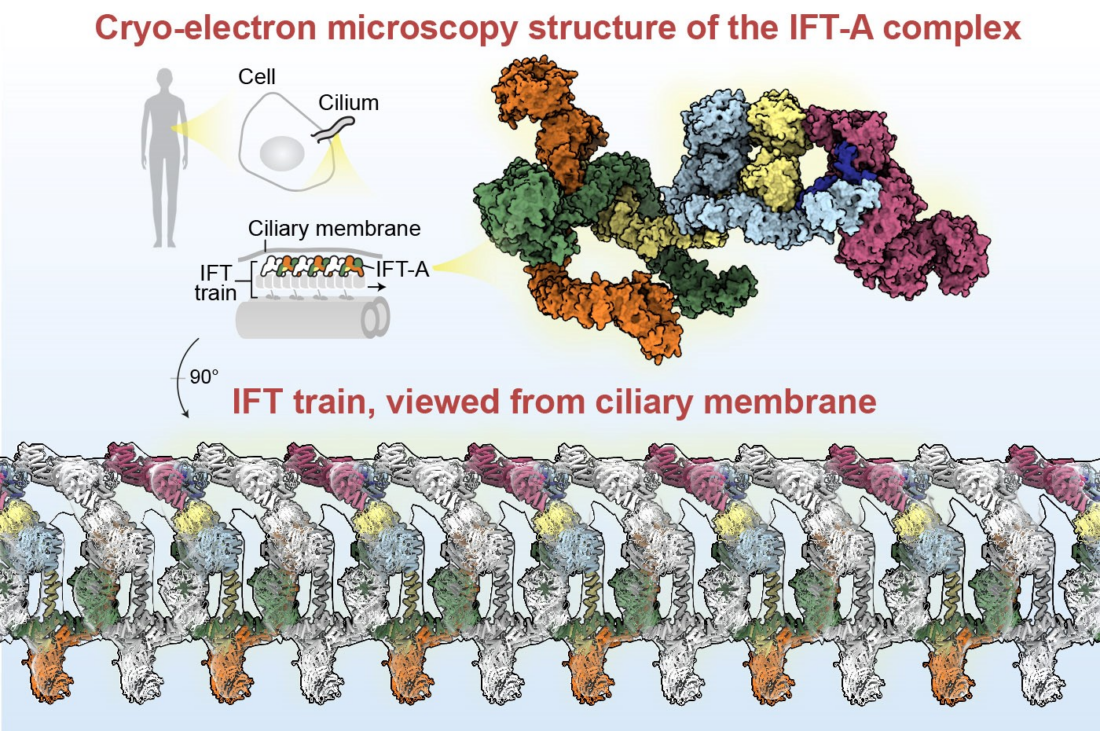
Eukaryotic cells have membrane-covered organelles on their surface called cilia, which act like antennae – sensing and transducing signals and beating to allow fluid flow and cell movement. Vital for cilia function are intraflagellar transport (IFT) trains, which are very large protein assemblies that act like molecular locomotives – trafficking cargoes into and out of the cilia. IFT is essential for the proper functioning of cilia and therefore essential for human life. Partial loss of cilia function causes human diseases called “ciliopathies” which range from vision impairment to skeletal abnormalities.
An IFT train is a polymer of two large protein complexes (IFT-A and IFT-B) and motor proteins, totalling around 1,000 proteins. Therefore, each IFT train is extremely large and complex, making it a challenging system to study and understand at the molecular level. Here, Hesketh & Mukhopadhyay et al. reconstituted and obtained high-resolution cryo-EM structures of the human IFT-A complex for the first time. Interestingly, known mutations in ciliopathies were identified at the interface of key protein-protein interactions. The structure was used to design and generate genetically modified cell lines that disrupt protein-protein interactions between IFT components. These experiments revealed how IFT-A binds to a class of adaptor proteins, called TULPs. The IFT-A:TULP interface was shown to be essential for the transport of multiple classes of membrane proteins into cilia, in addition to the ICK kinase that regulates the change in direction that IFT trains undergo at the cilia tip.
Together, these data shed light on the role of IFT-A in the train, and serve as a basis for future work to further understand the molecular intricacies of cilia transport systems. This is the latest paper published by the group of Anthony Roberts, who will be moving his group to the Dunn School in the spring of 2023.
Sophie J. Hesketh, Aakash G. Mukhopadhyay, Dai Nakamura, Katerina Toropova, Anthony Roberts (2022) IFT-A structure reveals carriages for membrane protein transport into cilia. Cell.
- Written by Isabella Maudlin (Murphy lab) @BellaMaudlin
Explore more
Read the paper
IFT-A structure reveals carriages for membrane protein transport into cilia.
Roberts Group
The Roberts group investigate the mechanisms by which motor proteins generate movement and spatial organisation within living cells. They are also interested in how defects in these mechanisms cause human pathologies.
Cell and Developmental Biology
Several Dunn School groups investigate the mechanisms underlying a range of important developmental and cellular processes such as signalling, transcriptional control, cell division, protein trafficking, and genome maintenance.