Novel insight into transcriptional functions of RNase H2 furthers our understanding of Aicardi Goutières Syndrome pathology
Published in Nature Communications, work by the Gromak lab uncovers key new functions for RNase H2 during transcription, and provides molecular explanations for the pathology behind Aicardi Goutières Syndrome.
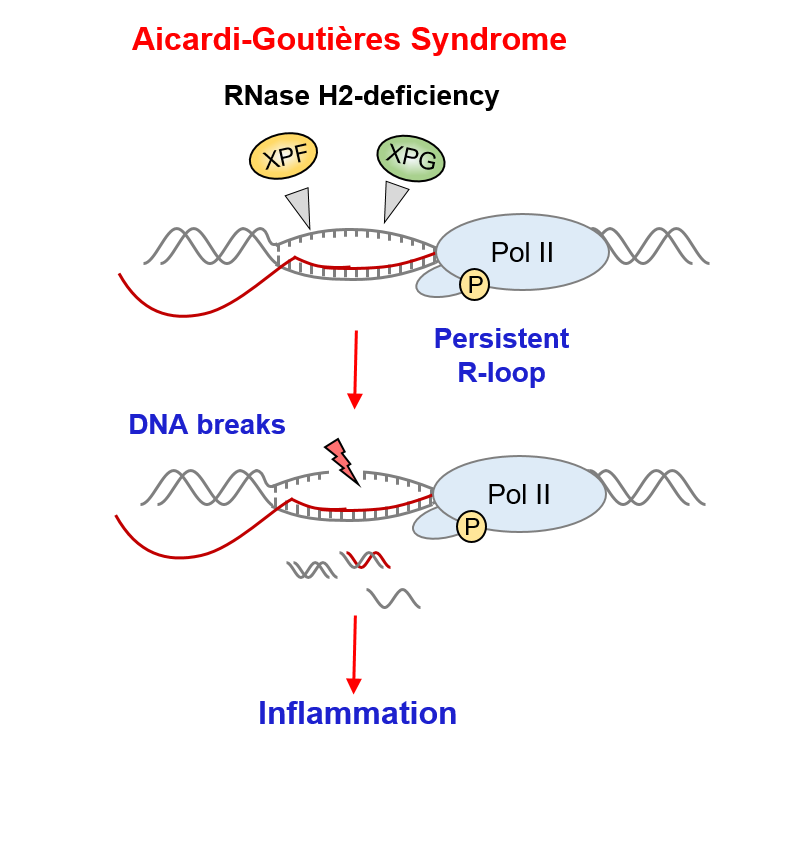
Aicardi Goutières Syndrome (AGS) is a severe neuroinflammatory disease caused by mutations in RNase H2 in more than 50% of the cases. RNase H2 is an enzyme that degrades RNA in RNA/DNA hybrids called R-loops. R-loops are formed during DNA replication and transcription, and their cellular levels need to be kept under tight control. Indeed, R-loops accumulation can cause replication-stress and genomic instability – both of which are linked to neurological disorders and cancer. Despite RNase H2 mutations causing more than 50% of AGS cases, the potential functions of RNase H2 outside DNA replication have until now remained unclear.
Natalia Gromak’s group have furthered our understanding of the basic molecular mechanisms that underly AGS pathology. Characterising the DNA replication-independent functions of RNase H2 during transcription, they find for the first time that RNase H2 interacts with RNA polymerase II, binds to actively transcribed genes, and degrades R-loops formed during transcription that exceed normal physiological levels. When RNase H2 function is lost, transcription is impaired leading to the accumulation of R-loops. These excess R-loops are recognised by endonucleases that cause DNA damage, triggering an inflammatory response that is characteristic of AGS.
Written by Isabella Maudlin (Murphy lab) @BellaMaudlin
Edited by Michael Tellier (Murphy lab) @Michael_Tellier
Article Title: Cristini et al. (2022). RNase H2, mutated in Aicardi Goutières Syndrome, resolves co-transcriptional R-loops to prevent DNA breaks and inflammation. Nature Communications, 13:2961 DOI : 10.1038/s41467-022-30604-0