ADP-ribosylation of DNA in vivo revealed for the first time
A study by the Ivan Ahel group reports the existence of ADP-ribosylation of DNA in vivo and its role in the regulation of bacterial growth. This research could have strong implications for the treatment of tuberculosis.
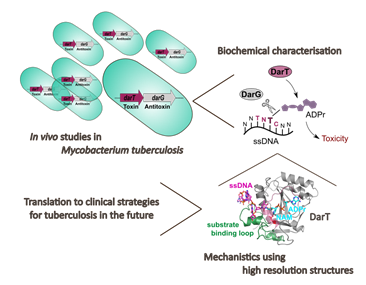
ADP-ribosylation is a modification of macromolecules found across all domains of life and regulates a variety of cellular processes including DNA repair and cell growth. A new study published in Nature reports for the first time that reversible ADP-ribosylation of DNA occurs in vivo. In a collaboration with Dr Rachel Butler and Professor Graham Stewart (University of Surrey), and Professor Tim Claridge (NMR facility), the Ivan Ahel laboratory was able to uncover the function of reversible DNA ADP-ribosylation in the human pathogen Mycobacterium tuberculosis as a signalling strategy for bacterial growth regulation. Understanding this aspect of M. tuberculosis may reveal opportunities for novel therapies. Dr Marion Schuller, who led the study, said “I am very proud to have been part of this exciting collaborative study since it provides a combination of new insights relevant for ADP-ribosyl transferase research, toxin-antitoxin systems and tuberculosis pathogenesis. Our efforts could reveal new mechanistic and evolutionary aspects for the class of ADP-ribosyl transferase enzymes but also build a fundamental understanding of the ADP-ribosylation of DNA.”
A new DNA damage-repair system
Reversible DNA ADP-ribosylation is catalysed on thymidine bases in a sequence-specific manner by the bacterial ADP-ribosyl transferase (ART) toxin named ‘DarT’. DarT is found in several bacterial species, including the human pathogens Mycobacterium tuberculosis, enteropathogenic Escherichia coli and Pseudomonas aeruginosa and is encoded in the DarT-DarG toxin-antitoxin (TA) system. Dr Schuller and colleagues showed that the DarT-DarG TA system acts a DNA damage-repair system whereby DarG can specifically remove the DarT-catalysed DNA ADP-ribosylation modifications. Furthermore, they reported the first high-resolution structures of DarT in pre- and post- reaction states, which allowed them to visualise ADP-ribosylated DNA for the first time, shedding new light on the molecular mechanism behind DNA recognition and ADP-ribosylation.
The regulation of bacterial growth by DNA ADP-ribosylation
Beyond induction of toxicity, the study also uncovered another role for reversible DNA ADP-ribosylation. In M. tuberculosis, the pathogen causing tuberculosis, it was shown that DarT preferentially modifies single-stranded DNA at the origin of chromosome replication which leads to growth arrest of the bacteria. This phenotype could be reversed upon removal of the DarT-catalysed ADP-ribosylation marks by DarG. These findings are corroborated by the fact that DarT and DarG are co-expressed with DnaB, the replicative helicase that localizes to the chromosome origin to open the replication fork. The scientists hypothesise that the DNA ADP-ribosylation marks could affect loading of DnaB helicase and other replication factors onto replication origins and, as a consequence, block chromosome replication. Together, these results reveal an exciting function for DNA ADP-ribosylation: the control of bacterial growth through regulating DNA replication and DNA repair.
A strategy to combat antibiotic resistance of M. tuberculosis?
The discovery of a new signalling pathway regulating bacterial growth could have important implications for targeting M. tuberculosis in the clinic and the development of new tuberculosis drugs. The induction of a slow and non-replicative growth state has previously been involved in antibiotic resistance. Dr Schuller and colleagues hypothesise that the activity of DarT could allow M. tuberculosis entry into this non-replicative growth state, making DarT a potential drug target. Furthermore, DarG was shown to be an essential gene in M. tuberculosis and considering the massive toxicity that is generally induced by DarT activity, targeting DarG with small molecule inhibitors could be a novel antibiotic approach.
Research article: Schuller, M., Butler, R.E., Ariza, A. et al. (2021). Molecular basis for DarT ADP-ribosylation of a DNA base. Nature 596:597–602
Written by Josephine Groslambert (Ahel lab)
Edited by Jake Burton (Dushek lab)