A changing view of how MPS1 initiates the Spindle Assembly Checkpoint
A new study published in Current Biology by the Gruneberg lab advances our understanding of the mechanisms by which cells correct mistakes during chromosome segregation.
3.8 million duplicated genomes have to segregate equally into daughter cells every second in our body to maintain normal cell and tissue function. Any problem in the chromosome segregation process can be detrimental, resulting in daughter cells having an abnormal number of chromosomes, a condition known as aneuploidy, strongly associated with cancer. Published in Current Biology, scientists from the Sir William Dunn School of Pathology now advance our understanding of the mechanisms by which cells correct mistakes during chromosome segregation.
New insights into how MPS1 is recruited to kinetochores
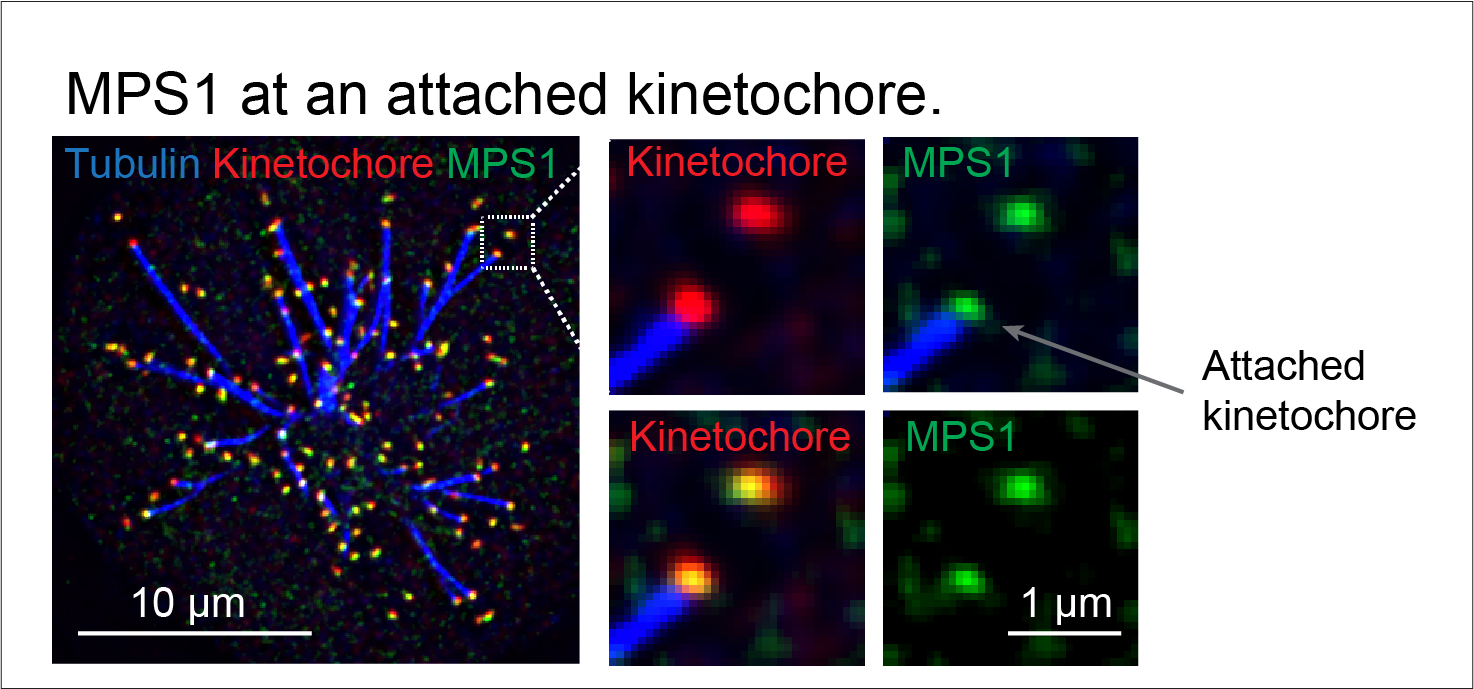
For chromosomes to segregate successfully, they must be captured in the correct orientation by spindle microtubules at chromosomal structures called kinetochores. The absence or incorrect attachment of microtubules to the kinetochore triggers signaling via the spindle assembly checkpoint. This checkpoint is essential to safeguard the fidelity of mitosis because its activation stops the progression of mitosis until all chromosomes are properly attached to microtubules. At the heart of this process is the kinase MPS1 which interprets the microtubule attachment state of the kinetochore and converts it into a decision to either continue or halt mitosis. “A key unanswered question is how MPS1 senses the state of the kinetochore, and whether this involves steric competition with microtubules for the same binding site on the kinetochore or a signaling-based mechanism.” Prof Ulrike Gruneberg, the corresponding author of this study, explained.
The Gruneberg team now provide evidence for a signaling-based mechanism at the heart of MPS1 kinetochore recruitment. Using a range of genetically modified human cell lines generated by the Gruneberg lab and the cutting-edge microscopy at the Dunn School, the authors directly showed that under some conditions MPS1 can still localise to kinetochores when microtubules are present. Rather, the level of MPS1 on kinetochores is meticulously regulated by the balance between the kinase Aurora B and the phosphatase PP2A-B56. “When many proteins are involved in the same pathway and are spatially close, understanding their relationship can be very tricky but we really take a leap in this work.” Dr Daniel Hayward, the co-first author of this study and now an independent researcher at King’s College London, said.
The unknowns and possibility
“Understanding that MPS1 recruitment is enzymatically controlled is a really exciting advance which prompts a rethink of how the microtubule attachment status of kinetochores is sensed.”, said Emile Roberts, joint first author of the study. Professor Gruneberg’s lab is still actively investigating how microtubule binding may tip the balance between Aurora B and PP2A-B56 activities to affect the level of MPS1 at the kinetochore. “Our lab is very keen to understand the roles and dynamics of different proteins involved in the spindle assembly checkpoint pathway and how their dysfunction contributes to human disease. Feel free to contact us if any young scientists would like to solve this puzzle together with us” Ulrike enthusiastically said. Her lab is currently recruiting PhD students as part of the Dunn School PhD Studentship Programme.
Written by Isaac Siu-Shing Wong (Raff Lab) @ISiuShingWong.
Edited by Isabella Maudlin (Murphy lab) @BellaMaudlin
Daniel Hayward, Emile Roberts, Ulrike Gruneberg (2022). MPS1 localizes to end-on microtubule-attached kinetochores to promote microtubule release. Current Biology