The ‘DART’ side of transcription: New role for NSUN2 in the RNA-dependent DNA damage response
Published in Nature Communications, the work highlights the importance of RNA modifications and how the NSUN2 protein is involved in the cellular response to DNA damage.
Cells produce a type of RNA called DARTs (damage-associated RNA transcripts) near the sites of the most toxic type of DNA damage, double-stranded breaks. These RNAs can form R-loops, structures where RNA binds back to the DNA strand, leaving a displaced single strand of DNA. Such R-loops could be a double-edged sword: whilst they can facilitate DNA repair (e.g., by acting as binding platforms for repair factors), they can also cause further DNA damage (if single strand DNA remains exposed), increasing the risk of mutations, and are linked to various diseases including cancer.
Known for its role in adding methyl groups to RNA, NSUN2 influences RNA stability and its interactions with other proteins. Since similar proteins have known roles in repairing DNA damage where R-loops are present, and increased levels of NSUN2 are common in many cancers, this prompted the group to investigate the role of NSUN2 in DNA damage repair.
Employing a variety of state-of-the-art molecular biology techniques, including modified 5-AZA-chromatin immunoprecipitation and Oxford Nanopore Technology for direct RNA sequencing, the team demonstrated a clear interaction between NSUN2, DICER, and R-loops. The findings reveal that NSUN2 is essential for promoting the cleavage of R-loops by the DICER enzyme. When NSUN2 is depleted, cells accumulate DARTs and R-loops around break sites, and DNA repair is delayed. The authors also show that NSUN2’s methylation activity is essential for efficient repair via homologous recombination, a high-fidelity repair pathway. Their results suggest that inhibiting NSUN2 activity and its associated RNA modifications could offer promising therapeutic strategies for conditions linked to DNA damage and R-loop accumulation.
Overall, this work underscores the intricate relationship between RNA modifications and DNA repair mechanisms, showcasing how cells maintain genomic integrity under stress. By highlighting the role of NSUN2 and RNA modifications, the Gullerova lab have opened new avenues for potential treatments for diseases resulting from DNA damage, paving the way for innovative therapeutic approaches.
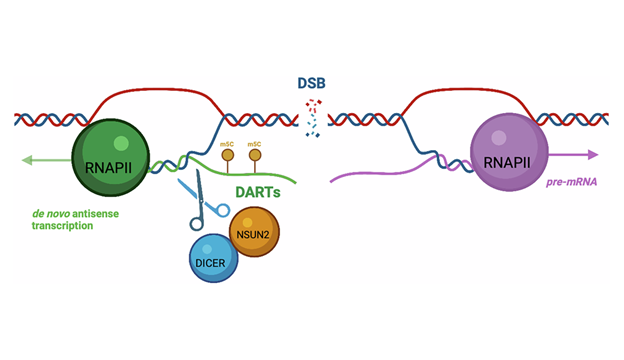
NSUN2 promotes RNA turnover at DNA breaks via DICER-dependent degradation of DARTs.
Written by Zara Kaplan (Freeman lab)
Explore more
Read the Paper
Alagia, A. et al (2025). NSUN2 facilitates DICER cleavage of DNA damage-associated R-loops to promote repair. Nature Communications, 16(1), 7882. https://doi.org/10.1038/s41467-025-63220-9
Gullerova Group
Understanding how intronic gene silencing is de-regulated in cancer cells and how synthetic tsRNA can be used therapeutically.
RNA & Gene Expression
Several Dunn School groups use a range of molecular biology approaches to investigate the fundamental mechanisms underlying transcription, transcriptional gene silencing, and RNA processing.